Aluminum alloy has low density, high fatigue resistance, high specific strength and specific stiffness. It is one of the non-ferrous metal structural materials widely used in industry. It has good corrosion resistance and is more and more used in marine engineering. widely.
Aluminum alloy has low density, high fatigue resistance, high specific strength and specific stiffness. It is one of the non-ferrous metal structural materials widely used in industry. It has good corrosion resistance and is more and more used in marine engineering. Extensive. The accumulation of foreign aluminum alloy real sea exposure corrosion data began with the exposure experiments in the Panama Canal area in the 1940s.

5052 aluminum alloy
From 1962 to 1970, the U.S. Navy carried out large-scale real sea exposure experiments in Port Wynemee, California.
The exposed materials include as many as 475 kinds of alloys, including 7 kinds of aluminum alloys such as 1100, 2014, 3003, 5052, 5083, 6061 and 7002. Not only the corrosion exposure experiments in shallow seas have been carried out, but also the deep sea conditions in the range of 700-2000m have been carried out. Study on the corrosion performance of the following materials.
| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Others: Each |
Others: Total |
Al: Min. |
| 5052 | 0.25 | 0.40 | 0.10 | 0.10 | 2.2~2.8 | 0.15~0.35 | 0.10 | – | 0.05 | 0.15 | remainder |
| 6061 | 0.4-0.8 | 2.2-2.8 | 0.15-0.40 | 0.1 | 0.45 | 0.04-0.35 | 0.10 | 0.15 | 0.05 | 0.15 | remainder |
Alloying elements in aluminum alloys mainly affect their candle resistance by forming different inclusions and alloys [M, 11, 14-22].
The main formation of 5052 aluminum alloy is Al6 (Fe, Mn) intermetallic compound and P phase (Mg2Al3). The potential of Al6 (Fe, Mn) intermetallic compound is about -700mV (psSCE, the same below), which is slightly higher than that of the matrix The potential (-800mV) exists as the cathode phase; while the Lu phase (Mg2Al3) potential is about -920mV, which is slightly lower than the potential of the matrix, acting as the anode 116_181.
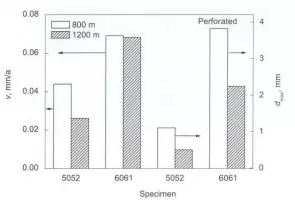
Corrosion rate of 5052 and 6061 aluminum alloys in deep sea
For 6061 aluminum alloy, the main formation is the impurity phase A1Fe-Si and Mg2Si phase. The potential of the A1Fe-Si phase is about -200mV, which is much higher than the potential of the matrix, and exists as a cathode phase; the potential of the Mg2Si phase is about -1200mV, far below the potential of the substrate, acts as the anode 119_221.
Both these second phases and the aluminum alloy matrix constitute a microcouple. The 5052 and 6061 aluminum alloys have different microcouple effects due to their different microstructures. The low-potential Mg2Al3 phase in the 5052 aluminum alloy is preferentially corroded as the anode, and the A1- The Fe-Mn phase has a high potential relative to the substrate, which will promote the corrosion of the surrounding substrate. Since the second phase of Mg2Si, which has a low potential in 6061 aluminum alloy, has a very low potential relative to the substrate, it acts as an anode and corrodes preferentially; the Al-Fe^Si phase Relative to the substrate potential is very high, as the cathode phase, it will promote the corrosion of the surrounding substrate, and accelerate the corrosion of 6061 aluminum alloy.

5mm thickness 6061 aluminum alloys
Compared with 5052 and 6061 aluminum alloys, the two phases in 6061 aluminum alloys have a larger potential difference, and the effect of microcouples is greater, which is prone to pitting corrosion. After pitting corrosion occurs, it will grow rapidly and eventually cause corrosion perforation. .The potential difference between the two phases in the 5052 aluminum alloy is smaller than that of the 6061 aluminum alloy, and the microcouple effect is small, and its pitting degree is slightly lower than that of the 6061 aluminum alloy. Changes in corrosion performance.
The ignition of aluminum alloys can be divided into two stages, that is, the pitting nucleation stage and the pitting growth stage. There are three main theories about the reasons for the rupture of the passivation film and the metastable pitting nucleation, namely, the ion penetration mechanism, Passivation film rupture mechanism and adsorption mechanism Among them, the chloride ion penetration mechanism is considered: when aggressive anions (such as C1″) are adsorbed on the passivation film, the chloride ion radius is small and easy to pass through the passivation film. Strong induction ions Conductive, so a high current density can be maintained at a certain point on the film, and the cations are promoted to move randomly. When the electric field at the interface of the film solution reaches a certain critical value, pitting corrosion occurs [241. The mechanism of passivation film rupture is Macxloiiald’s point defect model was developed: Aggressive anions (such as Cl_) are adsorbed on the oxide surface and react with cations at the ion/solution interface, resulting in the formation of cation vacancies in the passivation film. These cation vacancies move toward the metal/film interface Transport, when the cation vacancies reaching the metal/film interface cannot be consumed, the excess cation vacancies gather to form “vacancy clusters”, which effectively separate the passivation film from the metal substrate, forming pitting and the adsorption theory is called pitting corrosion. The occurrence is caused by the competitive adsorption of Cl_ and O126]. Once the corrosion pit is formed, the pitting corrosion develops rapidly. This is due to the acidification and autocatalysis process inside the pit: The state (potential is more negative) becomes the anode. The surface of the aluminum alloy outside the hole is still in a passivation state, and the potential is more positive to become the cathode; therefore, an activation-passivation micro-point couple corrosion cell is formed inside and outside the corrosion hole; the metal in the corrosion hole The anode reaction in the pores can be expressed as the accumulation of corrosion products (A1203, SiO2, and a small amount of Mg3(S04)2(0H)2 and NaCl) at the mouth of the corrosion pit, gradually forming a blocked cell, which hinders the formation of the pores. The migration of internal and external ions and the diffusion of dissolved oxygen. An oxygen concentration battery is formed inside and outside the hole; the high acidity and high chloride ion concentration environment in the hole promotes the further corrosion of the metal in the hole, thus forming an autocatalytic process of the blocked battery.
Factory hot selling thick aluminum foil, thin aluminum foil, household packaging aluminum foil, food packaging foil, medicinal aluminum foil
7075 aluminum sheet is the strongest heat-treatable alloy, introduced by Alcoa in 1943, and can be heat-treated to achieve strength levels comparable to many steel alloys.
Painted aluminum coil (Coated aluminum coil) is a popular material choice for construction, transportation, and signage due to its lightweight, durability, and versatility.
Embossed aluminum sheet is an aluminum product that is rolled on the basis of pure aluminum plate or aluminum alloy plate to form various patterns on the surface.
5052 aluminum sheet is part of the 5000 series of aluminum. The grades in this series are alloyed with magnesium (2.5%) and have medium to high strength properties.
5454 aluminum circles have all the properties of 5454 aluminum alloy and are often used in can sealing, non-stick pot bottoms, traffic signs, lampshades, etc.
No.52, Dongming Road, Zhengzhou, Henan, China
Henan Huawei Aluminum Co., Ltd, One Of The Biggest Aluminum Supplier In China Henan,We Are Established In 2001,And We Have rich experience in import and export and high quality aluminum products
Mon – Sat, 8AM – 5PM
Sunday: Closed
© Copyright © 2023 Henan Huawei Aluminium Co., Ltd
Latest Comments
Dear Sir, Please offer your best FOB Prices specs are as under ALUMINIUM STRIP (AL=99.50% MIN) SIZE:450 X32 X6 MM. DIN EN 570 EN-AW 1050 A, QUANTITY=3400KG
Hello, Would you be so kind to offer item as follows: Coil 0,6х1250 (1000)mm EN AW-3105 5tons
Hello, Can you offer me Aluminium plates? Actally I need: 110mm x 1700mm x 1700mm 5083 H111 - 21 pcs Next year planed is 177 pcs
Świetny artykuł. Byłem zachwycony, że wyszukałem ten artykuł. Wielu osobom wydaje się, że mają rzetelną wiedzę na ten temat, ale często tak nie jest. Stąd też moje miłe zaskoczenie. Jestem pod wrażeniem. Zdecydowanie będę rekomendował to miejsce i częściej wpadał, aby zobaczyć nowe rzeczy.
requirement of aluminium strip